Can a Monoclonal Antibody Reduce the Risk of Common Lung Infections?
Synopsis
Key Takeaways
- Nirsevimab can reduce bronchiolitis hospitalizations by 50%.
- It is most effective in infants under six months of age.
- The WHO endorses its use alongside a maternal vaccine.
- Immediate administration after birth is crucial for effectiveness.
- This study provides valuable insights into RSV prevention strategies.
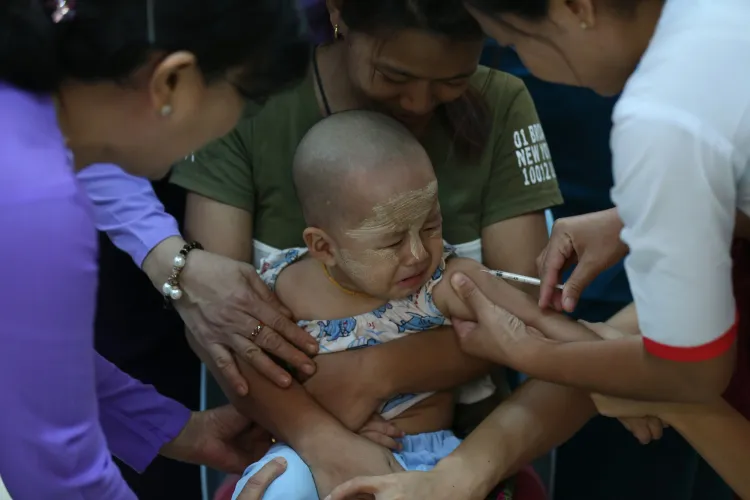
New Delhi, June 5 (NationPress) A single administration of the long-lasting antibody nirsevimab, designed to combat respiratory syncytial virus infections in infants, can lead to a 50% reduction in hospitalizations due to bronchiolitis, as revealed by a recent study.
Bronchiolitis is a severe viral infection that primarily impacts the respiratory system of children under one year of age, especially during the first six months, with increased incidence from November to March.
This illness is frequently linked to respiratory syncytial virus infection, occurring in approximately 75% of cases, and can result in respiratory failure, particularly in infants younger than six months.
In a groundbreaking approach, the study examined the tangible effects of nirsevimab by analyzing data from several European countries—Spain, the UK, and Italy—which have varying health policies.
Data gathered from 68 hospitals in Catalonia, Spain, as well as five hospitals in the UK and Italy, indicated that hospitalizations for bronchiolitis in infants under six months in Catalonia have nearly halved compared to averages from prior seasons.
Additionally, emergency room visits for this age group also saw a substantial decline. In contrast, no notable reduction was observed in other European regions where nirsevimab was not used.
The findings, published in the journal Lancet Regional Health – Europe, mark a significant advancement in evaluating the real-world effectiveness of novel preventive strategies against RSV, as stated by Danilo Buonsenso, a researcher in General and Specialist Paediatrics at the Catholic University, US.
Moreover, the study indicated that the impact of nirsevimab was less significant for older children aged six to 23 months, suggesting that its highest efficacy is found in the early months of life.
In May, the World Health Organization (WHO) urged all nations to utilize nirsevimab alongside a maternal vaccine—RSVpreF—to shield infants from the respiratory syncytial virus (RSV), a leading cause of acute lower respiratory infections in children worldwide.
While the vaccine can be administered during routine antenatal care, nirsevimab is delivered as a single injection of monoclonal antibodies, which begins to protect infants against RSV within one week of administration. Its effects last for a minimum of five months, covering the entire RSV season in countries experiencing RSV seasonality.
The global health organization recommends that infants receive a single dose of nirsevimab immediately after birth or prior to discharge from a delivery facility.