Could a New Implantable Device Rescue Diabetics from Severe Hypoglycemia?
Synopsis
Key Takeaways
- Innovative implantable device for Type 1 diabetes management.
- Eliminates the need for injections during emergencies.
- Automatically triggers glucagon release.
- Potential to aid diabetic children and during sleep.
- Can also administer epinephrine for severe allergies.
New Delhi, July 9 (NationPress) - A groundbreaking implantable device has been developed by a team of researchers in the US which aims to protect individuals with Type 1 diabetes from the risks of hypoglycemia, a condition characterized by dangerously low blood sugar levels that can have fatal consequences.
When glucose levels plunge critically low, it poses a severe threat to life, typically requiring the administration of a hormone called glucagon as a standard treatment.
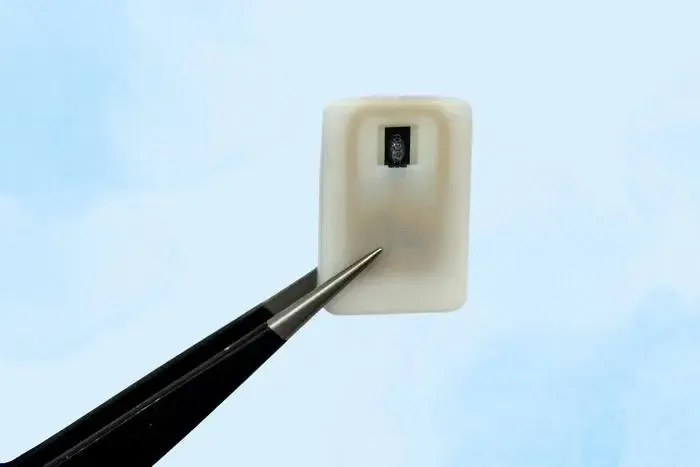
This innovative implant, designed by the Massachusetts Institute of Technology, contains a reservoir of glucagon that can be implanted beneath the skin and activated during emergencies, eliminating the need for injections.
Roughly the size of a quarter, the device features a compact drug reservoir constructed from a 3D-printed polymer. It can be activated either manually by the user or automatically via a sensor.
This technology can also assist in situations where hypoglycemia strikes during sleep, or for diabetic children who may struggle to administer injections independently.
“This is a small, emergency response device that can be implanted under the skin, ready to act when a patient’s blood sugar levels drop too low,” explained Daniel Anderson, a professor in MIT’s Department of Chemical Engineering.
“Our objective was to create a device that is consistently prepared to safeguard patients against low blood sugar. We believe this can significantly alleviate the anxiety surrounding hypoglycemia that many patients and their families face,” he added.
The device is capable of receiving wireless signals, and the drug release is activated by a glucose monitor when the wearer’s blood sugar falls below a predetermined level.
After testing the device on diabetic mice, researchers successfully triggered glucagon release as their blood sugar levels decreased. Within ten minutes of the drug being released, blood sugar levels stabilized, maintaining them within a normal range and preventing hypoglycemia.
In addition to its applicability for Type 1 diabetes, researchers have demonstrated that the device can also administer emergency doses of epinephrine, a medication used for heart attacks and to combat severe allergic reactions, including anaphylactic shock.









