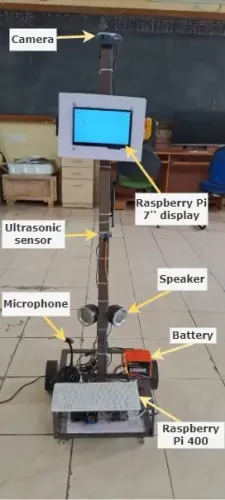
How Are Indian Scientists Enhancing CRISPR Protein for Gene Editing in Genetic Diseases and Cancer?
Synopsis
Key Takeaways
- GlowCas9 is a luminescent CRISPR protein developed to enhance gene editing.
- It allows real-time visualization of gene editing processes.
- The technology has potential applications in treating genetic diseases and cancer.
- GlowCas9 maintains stability at higher temperatures, improving treatment efficacy.
- It also hints at safe agricultural applications without genetic modification.
New Delhi, Dec 10 (NationPress) - Researchers at the Kolkata-based Bose Institute have developed GlowCas9, a groundbreaking CRISPR protein that illuminates during the gene editing process, enhancing treatments for genetic disorders and cancer, as announced by the Ministry of Science and Technology on Wednesday.
While the traditional CRISPR-Cas9 system was engineered to precisely cut and correct DNA, scientists faced challenges in visualizing the Cas9 enzyme, the 'molecular surgeon', in live cells in real-time. Conventional detection techniques, which require cell fixation or disruption, hinder the observation of these processes as they occur.
With the newly engineered CRISPR protein, researchers can now monitor the Cas9 enzyme, allowing for genome editing via the CRISPR-Cas9 mechanism to treat genetic diseases, including cancer.
The Ministry stated, “Gene therapy has the potential to serve as a permanent solution for numerous life-threatening hereditary conditions. However, developing efficient, economical, and safe gene therapy strategies has remained a significant challenge for many years.”
“Observing gene editing in action or witnessing the molecular machinery as it operates—cutting, repairing, and rewriting DNA within living cells—can facilitate the monitoring of CRISPR activities in live cells and tissues without causing damage,” it continued.
This study, led by Dr. Basudeb Maji from the Bose Institute, a research institute under the Department of Science and Technology (DST), represents a significant advancement in the visualization and monitoring of genome engineering.
Ph.D. researcher Arkadeep Karmakar, working in Maji’s lab, conceptualized GlowCas9, a luminescent adaptation of Cas9 that emits light within cells by merging Cas9 with a split nano-luciferase enzyme sourced from deep-sea shrimp proteins.
“These inactive enzyme fragments reconnect when Cas9 folds correctly, producing light. This occurs because when the pieces come into close proximity, they can reassemble, restoring their enzymatic function and generating a visible signal similar to the gentle glow of fireflies,” the researchers explained in their publication in the journal Angewandte Chemie International Edition.
The glowing activity enables scientists to monitor CRISPR actions in living cells, tissues, and even plant leaves without inflicting harm.
The team discovered that GlowCas9 is exceptionally stable, retaining its structure and functionality at elevated temperatures compared to conventional enzymes.
This stability is crucial for gene therapy, as reliable delivery of Cas9 can significantly enhance treatment effectiveness.
GlowCas9 also boosts the precision of homology-directed repair (HDR)—a vital DNA repair mechanism essential for rectifying hereditary mutations linked to genetic conditions such as sickle cell anemia and muscular dystrophy.
Furthermore, GlowCas9 can be traced in plant systems, indicating its potential for safe, non-transgenic applications in agricultural improvement, according to the researchers.